COVID Vaccine Technologies
This section is based on two video lectures from YouTube:
- All Types of COVIS-19 Vaccines, How They Work, Animation (Jan 28th, 2021) [219]
- The Technology Behind the New Covid-19 mRNA Vaccines (Feb 19th, 2021) [220]
There are different vaccine technologies: inactivated virus, subunit, viral-vector, mRNA.
| Technology | Examples |
|---|---|
| Inactivated vaccines | CoronaVac (Sinovc/China), |
| Covaxin (Bharat Biotech/India), | |
| Sinopharm BIBP, Sinopharm WIBP, | |
| TurcoVac | |
| Subunit vaccines | EpiVacCorona (Russia) |
| DNA/Viral-vector vaccines | Oxford-AstraZeneca (AZD1222, Covishield, Vaxzevria), |
| Johnson & Johnson Janssen (phase 3), | |
| Sputnik V (Gamaleya/Russia), | |
| Convidecia (AD5-nCOV, PakVac) China | |
| mRNA vaccines | Pfizer, Moderna |
-
Inactivated vaccine:
- This is the traditional vaccine, where the virus is grown in culture and then killed to destroy disease-producing capacity.
-
Subunit vaccine:
- Contains purified parts of the pathogen that are antigenic, or necessary to elicit a protective immune response. It is safer and more stable than vaccines containing whole pathogens.
-
Viral-Vector vaccine:
- Uses a modified adenovirus as a vector to deliver to a cell a DNA or an mRNA to produce an antigen for another infectious agent. [226]
- AstraZeneca [221] uses a modified chimpanzee adenovirus ChAdOx1. DNA codes SARS-CoV-2 spike protein, transcribes into mRNA, and translates into proteins. Uses HEK 293 embryonic cells from aborted fetuses [222]. Prohibited in South Africa, Switzerland, Denmark, Norway, because it causes low platelet counts (thrombocytopenia), blood clots, and haemorrhages. Has been reported to cause thromboembolic events, deep vein thrombosis, intravascular coagulation, pulmonary embolism, cerebral vein thrombosis.
- Sputnik V uses a human adenovirus serotype 26 on the first shot, and serotype 5 for the second.
- Janssen uses serotype 26. It is also associated with cerebral vein thrombosis, and low platelets counts. Forbidden in the European Union due to its adverse effects.
- Convidecia uses serotype 5. Its been used in Saudi Arabia, Mexico, Russia, Pakistan, Chile, Argentina.
- Uses a modified adenovirus as a vector to deliver to a cell a DNA or an mRNA to produce an antigen for another infectious agent. [226]
-
mRNA vaccines:
- A nano-lipid particle vector (NLP) delivers the mRNA to the target cell, where it gets translated into a viral antigen. When secreted by the MHC molecules, CD4 and CD8 T cells (part of the immune system) intercept it.
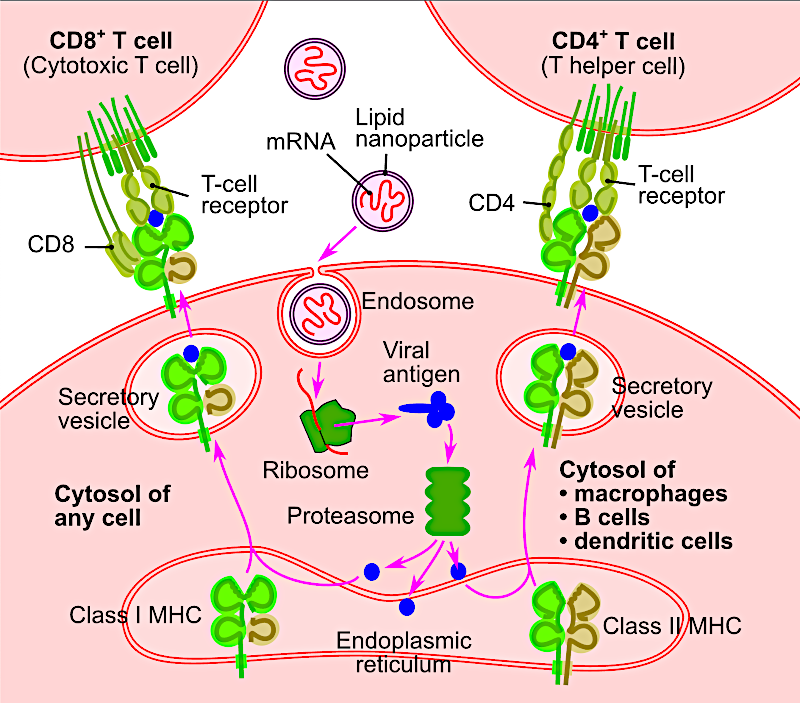
- Problems:
- Storage requires low temperatures: Pfizer -80 to -60C, Moderna -25 to -15C.
- The NLP includes PEG-lipid nanoparticles which cause allergic reactions and anaphylaxis [220]
- The NLPs do not stay in the deltoid muscle; they get transported intravenously, and through the lymphatic system and end up in different organs (such as heart, brain, kidneys) where the mRNA translation starts. On exit, the immune system attacks the spike protein, kills the host cell, potentially resulting in tissue damage and blood clots.
- A report, from Oct 2021, found that the spike protein doesn’t stay in the cytosol (inside the target host cell, outside of the nucleus), but enters the nucleus and inhibits DNA damage repair by impeding key DNA repair protein BRCA1 (cancer suppressing gene) and 53BP1 (double-strand break repair gene) to the damage site. This has implications for side-effects in full-length spike-based vaccines. [223]